Launch and Thrive.
Clinical Research Strategies helps early- and mid-stage life science companies begin on solid footing and stay poised for growth throughout their product life cycle.
TRANSFORMING INNOVATION INTO GLOBAL OPPORTUNITIES
The CRS team is comprised of accomplished PhD/RPh/PharmD scientists and bioengineers who have extensive problem-solving experience in contract research organizations, life sciences start-ups, academia, medical devices, and large pharma.
CRS distills the complexities of regulatory and research disciplines into accessible terms for life science researchers, executives, entrepreneurs, and investors alike who play a pivotal role in the development of devices, diagnostics, drugs, and biologics. Our goal is to improve our clients’ chances of clearing performance hurdles toward successful commercialization or exit.
Putting Clients on a Clear Path to Success
The mission of CRS is to improve business performance of early stage, start-up, and mature businesses with a successful clinical research development plan and strategy.
Our firm supports the entire product life cycle with competent leaders at a reasonable cost and unconstrained by corporate policy.
Project-specific, fit-for-purpose, ad hoc consulting, and staffing clinical trials is what we do best, no matter where companies are in their clinical development program.
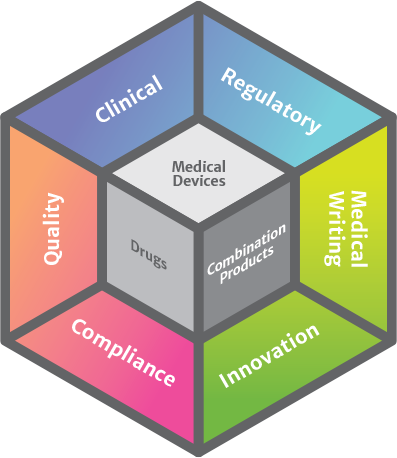
CRO services include project management, clinical monitoring, regulatory affairs, medical writing, quality assurance, and medical monitoring.
CRO & Sponsor Staffing
Make sure your clinical trial is on a steady and calculated course for success by avoiding cost over-runs and poor vendor performance.
Compliance, Governance & Due Diligence
Bring a watchful eye and sound decision making to your business for high performance and long-term sustainability.
Clinical & Regulatory Affairs
Put your products on a clear path from development to approval by eliminating hidden surprises and avoiding re-work later.
Risk Management & Quality Assurance
Implement continuous quality improvement and vigilance for your programs while mitigating the risk to your stakeholders.
Policy & Innovation
Manage the complexities of policy adherence and product development, and design your company policies to cultivate innovation.
Human Factors Studies
Before medical devices are introduced into the market, the use of human factors studies plays a vital role in ensuring that they will be effective and safe to use.
CRS has Special Contracts with DoD, NIH, DARPA, and others.
Solutions We Provide to Overcome Challenges
- Regulatory Pathway Assessments
- Effective and Efficient Clinical Strategy
- Filling Organizational Gaps
- Business Strategy Clarification / Partnering Opportunities Identification
- Quality Systems Creation / Updates
- EU MDR Compliance Work
- Real-World Evidence (RWE)
- Trial Master File Management and Inspection-Readiness
- First Article Development
- Grant Writing and Support Services
- Redaction Services for Public Domain Posting
- Human Factors / Usability Testing
- Due Diligence
- Trial Rescues
Areas of Expertise
Therapeutic Areas
Aging
Cardiovascular Disease
Central Nervous System
Critical Care Medicine
Gastroenterology, Genetics
Immunology
Infectious Disease
Men’s Health
Metabolic Disorders
Microbiome
Neonates & Pediatrics
Neurology
Oncology
Ophthalmology
Orthopedics
Pain
Precision Medicine
Radiology
Rare Diseases
Regenerative Medicine
Respiratory Diseases
Sleep
Women’s Health
Wound-Healing
Modalities
Biologics
Combination Products
Digital Therapeutics
In Vitro Diagnostics
Medical Devices
Small Molecules
Software as a Medical Device (SaMD)
HOW WE DEFINE SUCCESS
We are with our clients for the long haul — the ups and downs — supported by resilience and pragmatism.
The CRS team can help ensure a clear path to Success with a successful clinical research development plan and strategy.
Contact us today to arrange a no-obligation phone consultation.


