Recruitment Open for Contract Software Development Engineer – MEDICAL DEVICES
ABOUT CRS
We CARE about your BIG dreams and limited means.
We are with our clients for the long haul — the ups and downs — supported by resilience and pragmatism.
Clinical Research Strategies is a Pittsburgh, PA-based contract research organization and executive management consultancy for start-up and mid-size life sciences companies. We help instill fiscal discipline and reduce risks in clinical development, corporate affairs, and quality assurance programs.
Project-specific, fit-for-purpose, ad hoc consulting, and staffing clinical trials is what we do best, whatever stage your program is in.
CRS advisors have been everywhere – clinical research organizations, life science start-ups, and large pharma and medical device companies.
We can help you manage and overcome complicated trial operations and the burdensome regulatory pathways to a successful product launch.
OUR MISSION
To improve your business’ performance and provide a successful clinical research development plan and strategy.
Our guiding principles are:
INTEGRITY
TRUST
COMPLIANCE
TRANSPARENCY
AUTHENTICITY
PURPOSE

CRS Ranks #34 in the 2023 Pittsburgh Business Times List of Life Sciences / Medical Device Companies
The region’s biotech ecosystem remains strong in Pittsburgh — fueled by the presence of UPMC, Highmark/Allegheny Health Network and fed by the University of Pittsburgh, Carnegie Mellon University, Duquesne University and other regional institutions — as is evidenced by the companies on the List of Life Sciences and Medical Device Firms in the Pittsburgh region.
We’d Love to Learn about Your Program.
MEET THE CRS TEAM
Photo: Jen Worley
DAVID LINK, MBA
CEO and Chief Quality & Regulatory Officer
READ BIO
For more than three decades, David has been an innovative and transformative leader with progressive accomplishments in Quality Engineering, Quality Assurance, and Quality Management for market-leading technology and medical device manufacturers. He brings to CRS a proven track record of assuring customer quality satisfaction and improving profitability by identifying critical priorities, marshaling commitment, and managing cross-functional teams to successfully execute. David is results-driven by solving complex problems, driving change, and fostering and enabling quality culture for learning and improvement.
Photo: Jen Worley
ALETHEA WIELAND
President, Chief Operating Officer & Founder
READ BIO
Alethea brings 30-plus years of expertise in analyzing the intersection of healthcare innovation and policy; providing flexible clinical trial resourcing; developing sustainable corporate affairs programs and policies; training and managing resilient, high-performing clinical operations teams; mitigating risks of clinical trials; and facilitating transparent, accountable sponsor-CRO partnerships. She is the recipient of the 2017 Governor Tom Wolf Recognition in PA Life Sciences, 2017 Women in Bio P.O.W.E.R. Award, and 2017 Pittsburgh City Council Recognition in Life Sciences.
CANDICE BRETT
Manager of Business Operations
READ BIO
Candice earned her bachelors of science (BS) in business management from North Carolina State University. She has held various roles with increasing responsibility, including in financial consulting, billing, bank reconciliation, and financial reporting for small, mid-size, and large companies. Candice and her family spend significant time giving back to their community, including with food banks and other mentoring for those less fortunate. Ms. Brett lives in North Carolina with access to numerous CRS clients, including emerging life sciences companies and other existing partnerships.
Photo: Jen Worley
ANNA CARPIO
Clinical Research Associate
READ BIO
Anna earned a bachelor’s degree in psychology Cum Laude with Honors in 2023, from the University of Pittsburgh, and established an interest in neuroscience research with brain injury patients. Anna worked for Dr. Anthony Kline at the Safar Research Center, where she studied the effects of pharmaceuticals on experimental traumatic brain injuries. Through this research, she had the opportunity to attend the SACNAS conference as a Travel Scholar and won an Outstanding Research Presentation award.
Ms. Carpio was published as a corresponding author in Experimental Neurology, for her research contributions to “A bridge to recovery: acute amantadine prior to environmental enrichment after brain trauma augments cognitive benefit.” She has experience as a clinical assistant in rehabilitating TBI patients, and as a peer specialist at Western Psychiatric Hospital, furthering her passion for neuroscience and psychology.
Photo: Jen Worley
JULIE CRAMER, PhD, MS
Director of Clinical and Regulatory Affairs
READ BIO
Julie earned her PhD from the University of Pittsburgh in Pathology, with a focus on intestinal stem cells and their link to intestinal cancer. When the opportunity came to join a local life sciences startup that had licensed a technology from the University of Pittsburgh, she embraced the challenge and quickly came to understand how it takes careful management of a cross-disciplinary team to navigate the often turbulent entrepreneurial waters.
Dr. Cramer then returned to her alma mater to work in commercial translation at the University of Pittsburgh’s Innovation Institute. Acting as liaison to the University’s close clinical partner, UPMC, Dr. Cramer managed diverse programs, including digital health and immunotherapy, to enable nascent technologies to mature into valuable intellectual property ready for licensing.
Photo: Jen Worley
VONYA EISINGER
Manager of Quality and Regulatory Compliance
READ BIO
Vonya earned a bachelor’s degree with honors from Cornell University in biological and environmental engineering, and a master’s degree from Case Western University in biomedical engineering. She began her career working to determine genes responsible for mucus production in cystic fibrosis patients, carrying out toxicity studies to determine lethal dose of novel antibiotics on mice, and developing acute lung infection mice models.
After several years in a senior research assistant role at Magee Women’s Research institute (MWRI) studying gene expression of nicotine in lung fibroblasts and differentiating embryonic stem cells, Ms. Eisinger joined Perryman Company and held several roles in quality assurance, most significantly within the medical device sector where she analyzed validation requirements, requests for information, documentation, and complaint handling under ISO standards. Ms. Eisinger also participated in ISO 9001, AS9100, ISO 13485, and customer audits.
Photo: Jen Worley
FRANK FALCIONE, EMBA
Senior Executive Quality and Risk Management Consultant
READ BIO
Frank has 30 years’ experience as a senior executive working in domestic and international regulatory compliance, inspection-readiness, regulatory negotiations, quality management systems, and new product development within start-up medical device companies, military applications, and diagnostic instrumentation.
Mr. Falcione has contributed more recently to the implementation of MDD Class IIb and III devices for CE approval, transition to EU MDR conformance, and FDA Class III Modular PMA submissions. Additionally, he led quality programs for inspection-readiness, on-site and virtual audits and inspections, and face-to-face regulatory meetings. He remains actively involved in teaching regulatory/quality and product risk management at local colleges and universities and contributing to the development of young entrepreneurs.
Photo: Jen Worley
KAYSIE FOUST
Senior Clinical and Quality Specialist/Auditor
READ BIO
Ms. Foust earned a bachelor’s degree in biochemistry and molecular biology from The Pennsylvania State University. Ms. Foust began her career working at a CLIA-certified molecular medicine laboratory handling complex molecular assays and systems. She prepared sequencing libraries while also testing and evaluating new reagents and controls. Her career growth enabled her to move into GLP genetic toxicology assays, quality assurance, and report generation.
Kaysie moved from North Carolina to Pittburgh to work at Noveome Biotherapeutics (formerly Stemnion Inc.) where as a quality control analyst II, she spearheaded cGMP and cGLP work that supported product development. Her next move was with Interpace Diagnostics as a senior laboratory technologist, followed by a position as specialty laboratory technologist at the University of Pittsburgh Medical Center (UPMC) Genome Center. Prior to joining CRS, Ms. Foust worked at ALung Technologies in Quality Assurance.
In her spare time Kaysie volunteers for Lake Erie and Presque Isle clean-up initiatives and is passionate about supporting animal shelters.
Photo: Jen Worley
HALEY FULLER, PhD
Clinical and Regulatory Affairs Scientist II
READ BIO
Dr. Fuller earned her doctorate of philosophy in bioengineering from the University of Pittsburgh’s Swanson School of Engineering while conducting extensive research in on-a-chip systems, multi-scale robotics, and precision medicine as a part of the Synthetic Biology and Biomimetics Laboratory of Warren Ruder, PhD.
Haley was a Cardiovascular Bioengineering Training Program T-32 Fellow and collaborated with interdisciplinary research teams at the University of Pittsburgh Vascular Medicine Institute and the UPMC Heart and Vascular Institute. In this role, Haley assisted with the engineering of organ-on-a-chip devices to understand and treat cardiovascular pathogenesis. She used these systems to evaluate drug-laden microrobot localization and drug release efficacy as a demonstration of microrobots as targeted drug delivery systems.
A pivotal point in Dr. Fuller’s research career was a stint as a bioprocess engineer at the National Institute of Allergy and Infectious Disease (NIAID), where she was instrumental in conceptualizing DoE and high-throughput experimental approaches for vaccine candidate purification process optimization. Her efforts resulted in the development of clinical manufacturing processes, a high-throughput resin screen, and data generation for peer-reviewed scientific manuscripts.
Photo: Jen Worley
SHEILA GRAB, PhD
Clinical and Regulatory Affairs Scientist Consultant
READ BIO
Sheila earned her PhD in Pharmaceutical Sciences from the University of Pittsburgh’s School of Pharmacy, and Bachelor of Science degree from Pennsylvania State University. She has a breadth of experience in pre-clinical, clinical, and translational research focused on device, drug, biologic, and combination products for a range of indications. Additionally, she brings our clients more than five years of extensive experience in clinical trial management, regulatory submissions, pharmaceutical formulation, manufacture, characterization, and general laboratory procedures. Dr. Grab is highly adept at management consulting by juggling a variety of projects using her skills in scientific writing for SOPs, protocol development, grants, Investigator Brochures, Instructions for Use, and various other clinical-related documents, as well as regulatory research for US and international submissions. She serves as vice-chair of Young Women in Bio (YWIB) Pittsburgh Chapter and as a Women in Bio (WIB) Pittsburgh Chapter Member.
Photo: Jen Worley
ALYSSA HARRIS, MS
Manager of Clinical and Regulatory Affairs
READ BIO
Alyssa serves as Manager of Clinical and Regulatory Affairs at CRS and is responsible for managing multiple trials involving medical devices and drug development research in critical care, GI surgery, abdominoplasty, oncology, infectious diseases, respiratory disorders, and digital therapeutics. Ms. Harris also has experience managing federally funded research programs in vulnerable populations, specifically in the neonatal intensive care unit.
Alyssa earned her master’s degree from the University of Pittsburgh in Health, Physical Activity and Chronic Disease, focusing on the research track. Her undergraduate degree is in Exercise Physiology, where Ms. Harris gained extensive experience working in chronic disease research, specifically with metabolic disorders, and leading weight loss and physical activity interventions. Ms. Harris brings a unique range of expertise to CRS, and thrives in an environment that uses new methods and tools in research.
Photo: Jen Worley
YUKI KINOSHITA
Clinical Research Associate
READ BIO
Yuki earned a bachelor’s degree in public health with a clinical trials research concentration, magna cum laude, from Kent State University in May 2020, with membership in the Alpha Lambda Delta Collegiate Honor Society. She also received the University Award, President’s Scholarship, and Trustee Scholarship.
Prior to joining CRS, Yuki worked for ALung Technologies and LivaLova, Inc. in clinical operations positions where she gained experience working on trial master files (TMFs), literature reviews, and clinical evaluation reports (CERs) for medical devices. She is dedicated to contributing to the CRS team and growing her skills in clinical research. In her spare time, Yuki enjoys pilates, hiking, and meditation.
Photo: Jen Worley
SAMANTHA KREBS
Clinical Research Associate
READ BIO
Samantha earned a bachelor’s degree in biobehavioral health from Pennsylvania State University (PSU). She took focused coursework in Clinical Research Practice, Health Promotion II, Anatomy and Physiology II, Chemistry Principles II, Epidemiology, Genetics, Statistics, Research Applications, Diversity and Health, Bioethics, Kinesiology, Nutrition, and Psychology.
Samantha completed numerous certifications in the research and medical field, including: First Aid & CPR and Mental Health First Aid; CITI Programs Biomedical Human Subject Research (IRB) Course, FDA-Regulated Research, OSHA Bloodborne Pathogens, Shipping of Regulated Biological Material; and, Professional Development Certification for Center-Based Care (Building Blocks for Quality). Her leadership skills were honed by chairing the 2021-22 THON and coaching club field hockey. In her spare time, Samantha enjoys hiking, fishing, and anything outdoors.
Photo: Jen Worley
SIDNEY LANE, PhD
Clinical and Regulatory Affairs Project Lead
READ BIO
Dr. Lane earned a doctorate in Microbiology and Immunology from the University of Pittsburgh, School of Medicine, conducting extensive research on immune dysfunction and host-microbe interactions during polymicrobial respiratory infections in the laboratory of Dr. Jennifer Bomberger, PhD.
Sidney’s research led to multiple peer-reviewed publications and fellowships, including an appointment as a TL1 Clinical and Translational Sciences Fellow. In this role, Sidney expanded her clinical research knowledge through coursework and a personalized training program, allowing her to grow her passion for life sciences research translation. Following her time as a TL1 Fellow, Sidney joined the CRS team as the inaugural intern in the newly established partnership between CRS and the University of Pittsburgh. Outside of the workplace, Sidney loves to play with her dog and enjoys yoga, bouldering, and staying up-to-date on her favorite home renovation shows.
JENNIFER LE, PHARM.D.
Clinical and Regulatory Affairs Project Lead II
READ BIO
Jennifer earned her doctorate of pharmacy from the University of Rhode Island, School of Pharmacy. She recently completed a 5-month long internship with CRS and landed her role by proving exceptional performance, know-how, and character.
Dr. Le currently serves as a project lead at CRS where she is involved in clinical trials for medical devices and drugs, regulatory submissions, and medical writing. She has experience managing clinical data, writing documents such as informed consent forms, preparing 510k submissions, and facilitating correspondences with the FDA. Dr. Le enjoys utilizing her pharmaceutical knowledge and critical thinking skills at CRS to provide exceptional clinical research services to our clients and continue fostering innovation.
Photo: Jen Worley
LAURA LUND, PhD
Senior Executive Management Consultant
READ BIO
Dr. Laura Lund is an accomplished medical device development and commercialization expert, having spent significant time in the industry as a mechanical bioengineer, including extensive experience in global regulatory and clinical strategy, both pre- and post-market. She has expertise in journal publications, regulatory submissions, sales and marketing materials, IP development, training materials, investor decks, collaborations with medical advisory boards, and presenting at conferences. She is well-versed with the design, implementation, and regulatory requirements for pre-clinical in vitro and in vivo testing.
Dr. Lund’s therapeutic areas include drugs, biologics, and medical devices in the following areas: critical/intensive cardiopulmonary care, mechanical ventilation, ECMO, ECCO2R, artificial hearts (LVAD, RVAD, TAH), intra-aortic balloon pumps, central venous catheters/cannulation, continuous dialysis, high-pressure contrast injection, imaging, digital therapeutics, COVID-19 in vitro diagnostics; COPD, ARDS, acute respiratory failure, asthma, cystic fibrosis, heart/lung transplantation; Digital Therapeutics, Software as a Medical Device (SaMD), In vitro Diagnostics (IVDs); 3D bioprinted regenerative medicine; central nervous system (CNS); oncology; and many more.
Photo: Jen Worley
MATT SUNDERMAN, PhD
Senior Clinical and Regulatory Affairs Scientist
READ BIO
Dr. Sundermann earned an undergraduate Biomedical Engineering degree from Vanderbilt University in 2011 and a PhD in Bioengineering from the University of Pittsburgh in 2017. Prior to joining CRS, Matt held a Senior Clinical Strategy Manager role at Smith and Nephew and a Clinical Research Scientist role at ZOLL Medical Corporation. Between 2012-2017, he was employed in the UPMC Artificial Heart Program as a Clinical and Flight Engineer.
Outside of work, Matt is active with the Explorers Club of Pittsburgh and volunteers as an instructor in the club’s backpacking, climbing, and mountaineering schools. Matt resides in the Lawrenceville neighborhood of Pittsburgh with his partner Kelly and labrador retriever named Summit.
Our Supplementary Team is Ready for Your Assignment!
Project Management Directors and Project Managers
- Experts from CRO, Sponsor & Site side
- ≥ 10-20 years’ experience in research and consulting services
- Cardiovascular, oncology, regenerative medicine, CNS, women’s/men’s health, implantable devices, vaccines, infectious diseases, GI, metabolic, immuno-oncology, respiratory, rheumatology, nephrology, rare diseases… and many others
Senior Clinical Research Associates / Auditors
-
Experts from CRO, Sponsor & Site side
-
≥ 20-30 years’ experience in research and consulting services
-
Cardiovascular, oncology, regenerative medicine, CNS, women’s/men’s health, implantable devices, vaccines, infectious diseases, GI, metabolic, immuno-oncology, respiratory, rheumatology, nephrology, rare diseases… and many others
Senior Scientist Executives
-
Experts from Sponsor & Government Firms
-
≥ 15-30 years experience in life sciences
-
Drug, biologic, device, IVD, Artificial intelligence / Machine learning, software as a medical device (SaMD), usability testing
Quality Assurance Engineers and Auditors
-
Site, Sponsor, Vendor auditors
-
Quality Management Systems (QMS)
-
483/Warning Letter response and mitigation
Regulatory Strategies & Submissions
-
FDA Regulatory Agent, Pre-IND, Q-Sub, Pre-IDE, IND/IDE, 513(g), 510(k), De Novo, PMA, NDA, BLA, Breakthrough, etc.
-
Health Canada
-
Technical Files and Design Dossiers
Scientific Writing
-
EU MDR Literature Reviews and Clinical Evaluation Reports
-
Protocols, Investigator Brochures, Technical Documents
Medical Experts/Medical Monitors
-
Medical oncologists, surgeons, intensivists, internists, primary care, most other specialties covered
LET’S TEAM UP TO GIVE YOU YOUR BEST SHOT AT A SUCCESSFUL LAUNCH
Contact us today for a free phone consultation.
The CRS Senior Management Team Helps Enable Growth and Awareness at the Local University Levels.
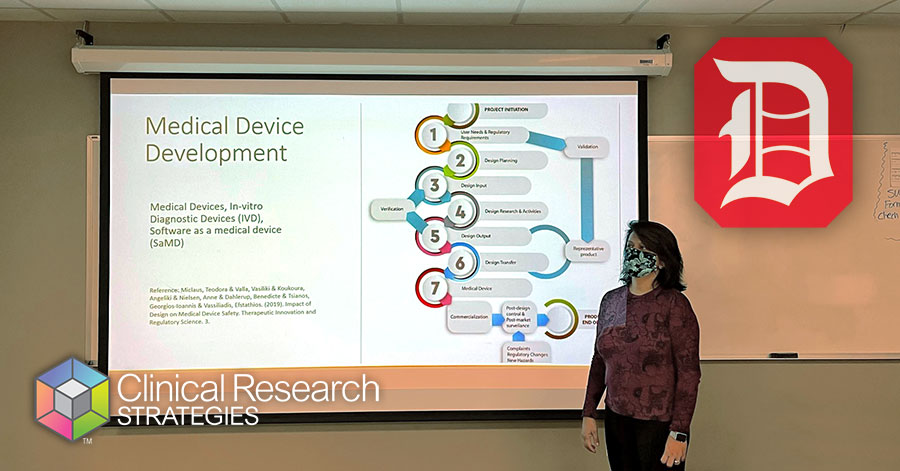
Parul Nisha, PhD, Senior Director, Clinical and Regulatory Affairs at CRS, making a difference and giving back as she presents her expertise to the next generation of Biomedical Engineering students at Duquesne University.
CRS is proud of our teammates, Julie Cramer, PhD, MS (judge) and Parul Nisha, PhD (judge), for their support of the Pittsburgh Regional Science & Engineering Fair at the Carnegie Science Center. Thank you for your civic involvement!
Julie Cramer, PhD, Associate Director of Clinical and Regulatory Affairs at CRS, presents a seminar to the University of Pittsburgh biomedical graduate students who are in the Cellular Approach to Tissue Engineering and Regeneration (CATER) program, funded by a T32 grant. Julie, a former member of the CATER program and Pitt grad student herself, says, “It feels great to give back!”
It was a great honor to be invited alongside representatives from Philips, Covestro, and ZOLL Medical Corporation to Leda Klouda, PhD’s Biomedical Engineering Department and the Biomedical Engineering Society Career Event at Duquesne University held on March 30, 2022. Our Clinical Research Strategies, LLC teammates, Brian Sullivan and Julie Cramer, were thrilled to provide highlights of their own career trajectories (education, first and subsequent jobs) and speak about our company’s products, market, and customers. Thank you, Leda Klouda, PhD and students!
CRS TEAM UPDATES
Congratulations to Anna Carpio, CRS Clinical Research Associate, for completing Barnett’s CRA & CRC Beginner Program!
Anna used this 10-week beginner course to gain an excellent introduction to clinical research and the job responsibilities of Clinical Research Associates (CRAs) and Clinical Research Coordinators (CRCs), exploring topics relevant to those considering a career as an entry-level CRA or CRC.
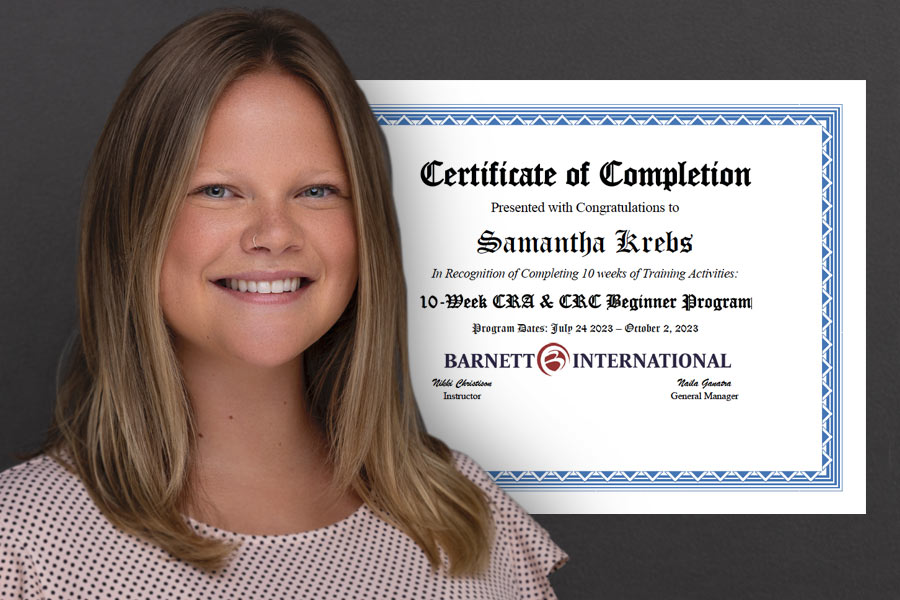
Congratulations to CRS Clinical Research Associate, Samantha Krebs, for completing Barnett’s CRA & CRC Beginner Program!
The 10-week program provides a comprehensive introduction to clinical research and the job functions of the Clinical Research Associate (CRA) and Clinical Research Coordinator (CRC) for drug, biologic, and device trials.
Samantha is using this training to further her studies in the field of life sciences and to grow in her role as a CRA.
CRS INTERN PROGRAMS
CRS has an Undergraduate Internship Affiliation Agreement with Penn State to provide students with real-world clinical research experience that supplements what they learn in the classroom. Their Special Topics course focuses on the practice of clinical research as a cornerstone to academic medicine and research, using novel approaches to treat disease while forging new best practices in clinical care. Together with the opportunity for internships, this course is a keystone part of Penn State’s Clinical Research Program.
CRS is also collaborating with the University of Pittsburgh School of Medicine Graduate Studies to provide interns with experience in clinical and regulatory affairs, medical writing, consulting, and business development. Interns will work with the CRS team to gain an understanding of clinical trials and the different steps involved in bringing new technologies to a place of impact.
CRS Welcomes Andrea Cruz!
CRS welcomes our newest intern, Andrea Cruz, a 5th-year pre-doctoral candidate at the University of Pittsburgh School of Medicine working towards a degree in cellular and molecular pathology. Broadly, her graduate work elucidates biological mechanisms that promote the initiation and progression of pediatric high grade gliomas and identifies novel biomarkers or targets to aid in the development of new diagnostic tests and chemotherapy drugs. She is passionate about clinical research consulting and aiding in the development of new technologies and therapies to ultimately improve human health outcomes.
Welcome to the CRS team, Andrea!
CRS Welcomes Alexis Duray!
CRS welcomes our newest intern, Alexis Duray. Alexis earned her Bachelor of Science at the University of Pittsburgh in Biological Sciences and History and Philosophy of Science. She is currently a graduate student in the Microbiology and Immunology Program at the University of Pittsburgh, working in the laboratory of Dr. John Alcorn at UPMC Children’s Hospital. The focus of her research is how infection with respiratory viruses, particularly influenza, can damage the host airways and lead to increased incidence of secondary bacterial pneumonia. Additionally, she is working on a project to predict length of hospitalization in pediatric patients admitted to the hospital for respiratory infections.
Welcome aboard, Alexis!
CRS Congratulates Korrine McCutcheon!
CRS congratulates Korrine McCutcheon, a fourth-year student at Penn State Greater Allegheny pursuing a Bachelor of Science in Biobehavioral Health. Korrine is interested in clinical research in the disciplines of neuroscience and pharmaceutical development. During her 12-week internship, she was integrated into our Clinical Trials Team where she was able to apply what she learned in Penn State’s Clinical Research Program and gain real-world experience.
Korrine is thankful for her experience with CRS, stating, “Clinical Research Strategies provided me with meaningful and diverse experiences pertaining to clinical research. I learned and worked with various FDA guidelines. I now have a strong foundation on the guidelines for clinical research and how they promote efficacy and safety in clinical research. I was also able to experience the process of clinical trials in various stages. I am very appreciative of my internship and experience at CRS.”
Please join the entire CRS team as we congratulate Korrine!
CRS Congratulates Tracey Myers, Our Recent Intern!
CRS congratulates Tracey Myers, a PhD Candidate in the Center for Neuroscience at the University of Pittsburgh. Her graduate work focuses on the development of therapeutics for triosephosphate isomerase deficiency, an ultra-rare neuromuscular disease. Tracey is interested in clinical research and passionate about bringing novel therapies from bench-to-bedside.
Tracey appreciates her experience working with the CRS team, stating, “My internship at CRS was exceedingly motivating in my pursuit of a career in clinical and regulatory affairs. I gained substantial exposure to the process of clinical development, from conceptualizing clinical trial protocols all the way to creating clinical evaluation reports. This experience has greatly expanded my knowledge base and will continue to benefit me as I begin to consider career opportunities post-PhD.”
Please join the entire CRS team as we congratulate Tracey!
CRS Congratulates Olivia Moore, Our Recent Intern!
CRS congratulates our recent intern, Olivia Moore, a senior at Penn State Greater Allegheny, majoring in biobehavioral health. Her career goal is to be involved in clinical research. While at CRS, she was an integral part of our team, learning what clinical trial management involves, and a little about the associated regulatory requirements.
Olivia found her experience at CRS to be rewarding, stating, “This internship was incredibly educating and eye-opening to understanding the world of clinical research and all that it entails. Being a part of this internship has opened my eyes to different occupational opportunities that I may enjoy taking part of in the future.”
Please join the entire CRS team as we congratulate Olivia!